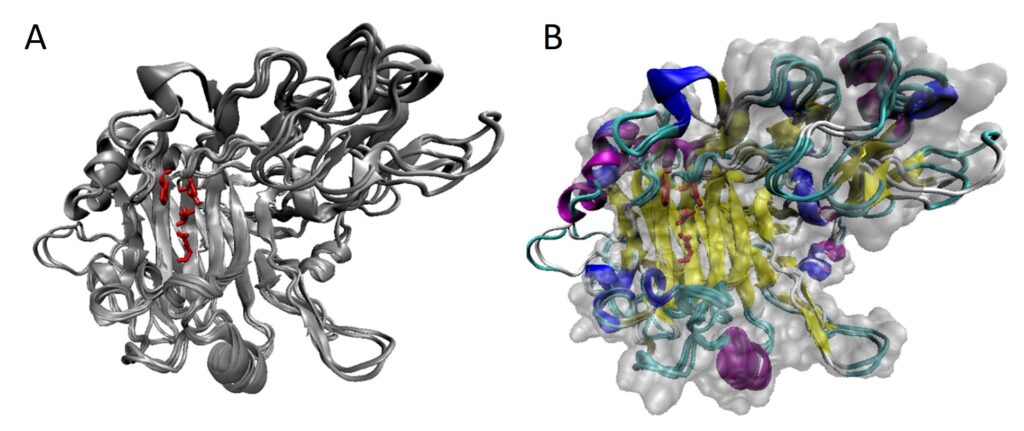
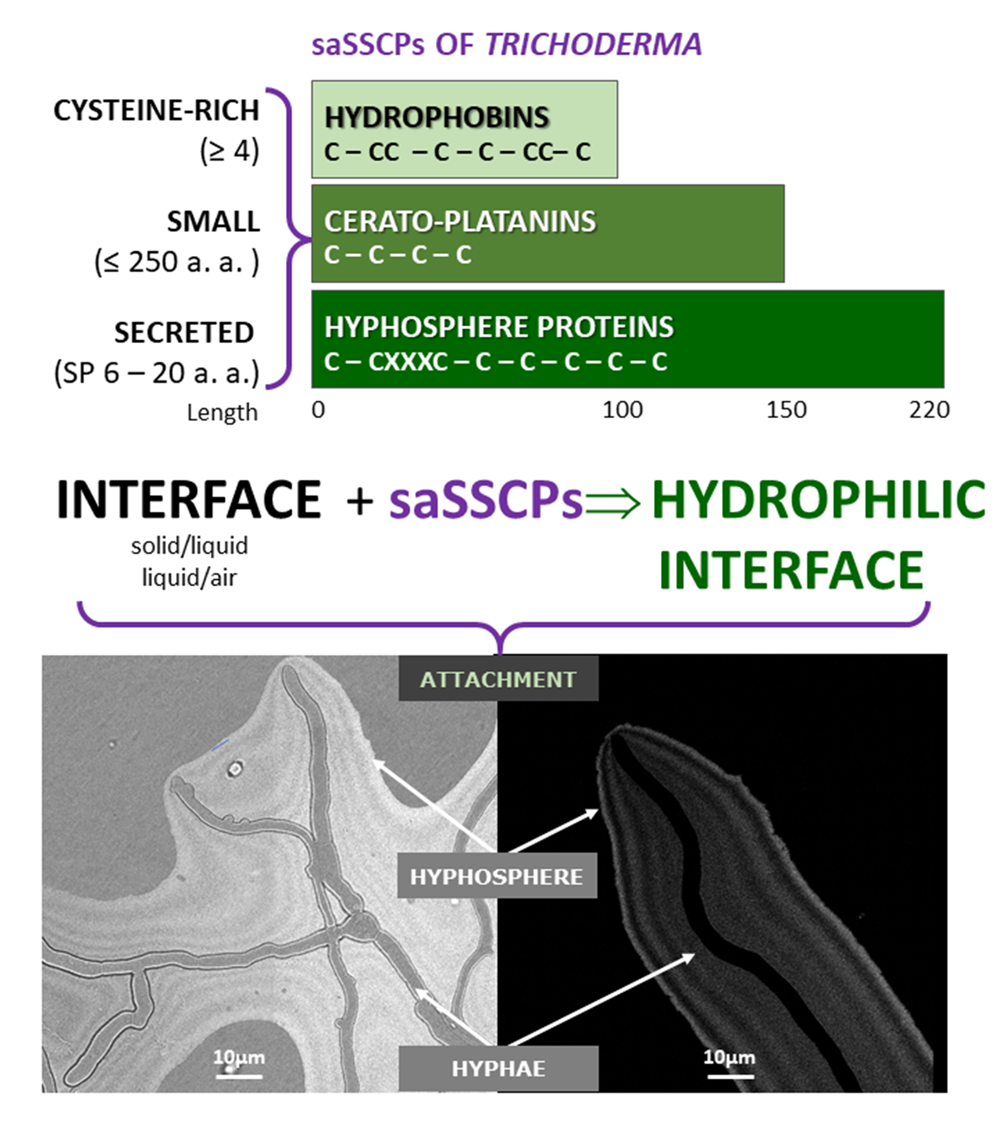
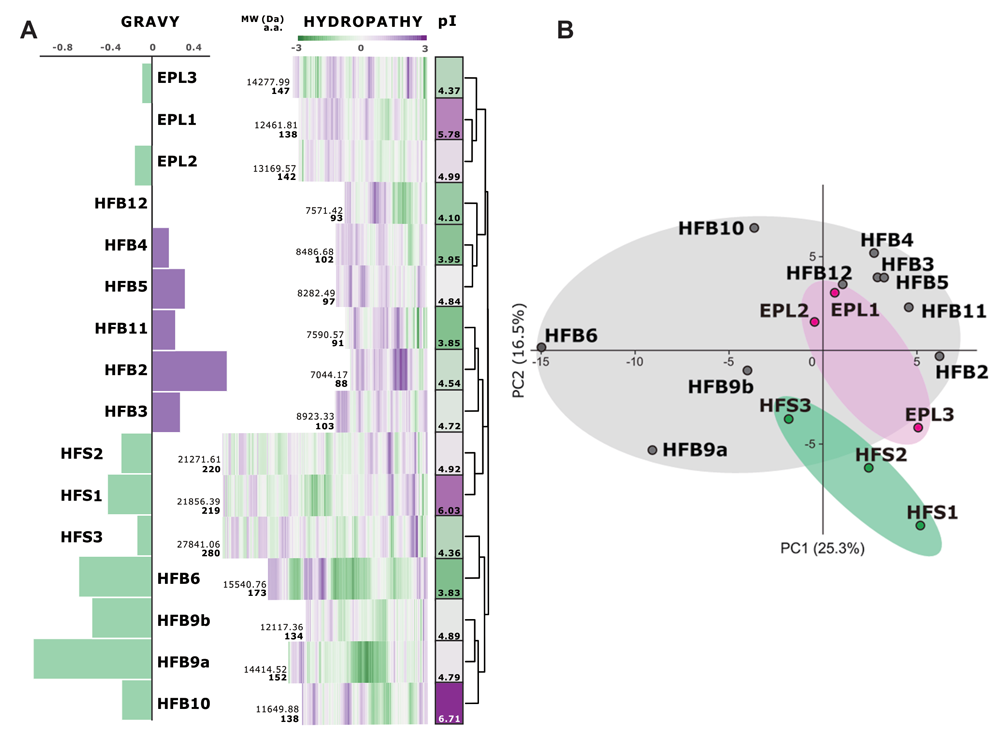
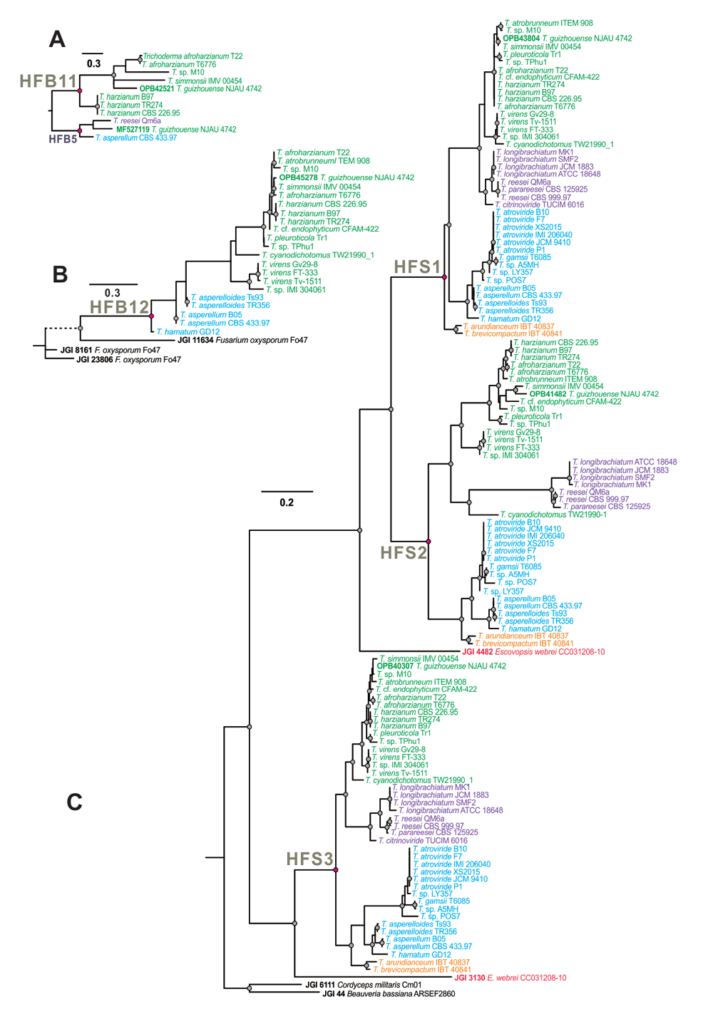
Winter semester 2021: Trichoderma genes!
Chinese Version: 中文
- personal tutoring
- individual schedule
- real scientific material
- useful outcome
- personal tasks
- up-to-date science
- no lectures
- no written reports
- no shared deadlines
- individual work
- online and remote
- optional group work
What will I learn?
- The broad scope of fungal functional genetics
- Fungal genes
- Genomics
- Gene nomenclature
- MycoCosm, KEGG, and other web resources on fungal genetics
- Recent literature on fungal genetics (incl. fungal diversity and applications)
- Research community
- Terminology
- Evolution, diversity, and speciation
How will it work?

- Register by sending a personal message to FungiG or contact “IrinaDruzhinina” on WeChat. Below please find the QR code.
- Get the first task (1/10 of the entire exercise).
- Submit your results and questions (Email or Wechat)
- Get feedback and answer to your questions.
- Repeat until the course is completed.
What is the content of the course?
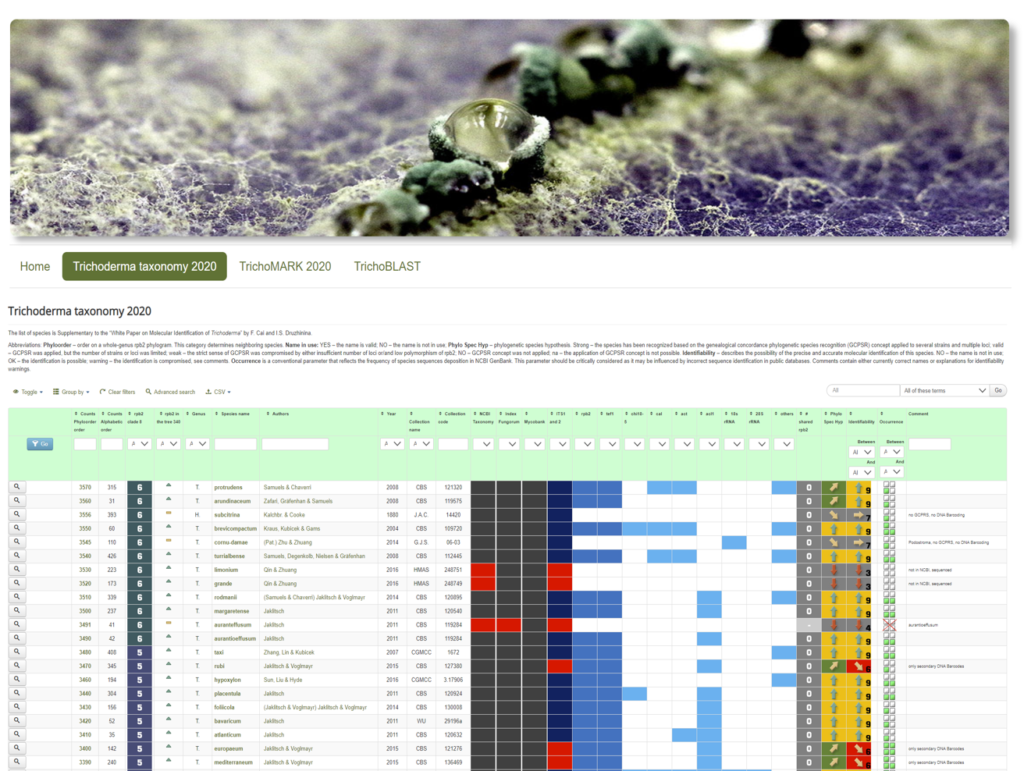
The WS2021 course will be based on the dynamic list of Trichoderma genes and Trichoderma-associated genes (mainly plant genes studied along with Trichoderma). The next courses may be based on other model fungi.
The tasks for this course are divided into small sets. Each time, a student will get his or her own random set of 2–3 fungal genes. The task will be to search for the genome IDs, protein IDs, function(s), evolutionary history, mutant(s), phenotype(s), GO term(s), KEGG group(s), genomic/chromosome location(s), cluster organization(s), functionally associated gene(s), published gene name(s), host genome(s), orthologue(s), paralogue(s), other homologue(s), patent(s), applied value(s), product(s), and reference(s).
The student is expected to systemically collect associated counts, such as the total number of publications, number of patents, number of Trichoderma spp. studied, etc. Some genes are intensively studied (e.g., cbh1, lae1), and the task will take more effort compared to the others that have only been published once.
What should I know before the course?
- Basic eukaryotic microbiology and basic mycology
- Basic biochemistry and cell biology
- English reading skills
- Advanced skills in retrieval of scientific literature (FungiG will provide help)
What is the main challenge of the course?
The concept of a fungal gene, gene definition, gene nomenclature in fungi, inconsistency in research approaches, the diversity of genes, the unequal quality of genome annotations, fungal diversity, and taxonomy.
What exactly should I do to complete the course?
A student is expected to deliver a table (as will be specified in the task) describing the functions and properties of several Trichoderma genes or genes associated with Trichoderma research.
The minimum set of genes is 25 (10 sets of 2–3 genes); the upper limit is 300*.
The advanced version of the course includes the joint (online/offline) seminar with students’ presentations and discussions. The aim of the seminar is to appoint the top ten most studied, most useful, and most controversial genes in Trichoderma, respectively.
* The total number of Trichoderma genes is ~12 000, but the number of genes studied for their function(s) is still meager.
Can my tasks be redundant to the tasks of my colleagues?
Sometimes, yes. The majority of the tasks will be unique. However, the genes used for the tasks that failed or were superficially performed remain in the pool of genes for the course.
Can I add genes to the list?
Yes. Students are welcome to do so. These can also be genes from other fungi that are not yet studied in Trichoderma. Please send your proposals to ISD.
What is the course language?
English.
What is the schedule of the course?
The schedule of the course is flexible. The results can be sent at any time. The feedback will be returned within 72 hours, or the exact time will be specified.
How long will it take? How deep can I go?
The course is designed such that an advanced Ph.D. student working on fungal genetics is expected to spend one day per week for 10 weeks or make it in a block (2 weeks, full time). The minimal workload corresponds to 80–90 working hours or 3 ECTS (European Credit Transfer and Accumulation System, Bologna Process).
As you progress, it should become faster. After you get in shape, you may either spend less time per week or learn more genes.
Can I do the entire course remotely?
Yes.
Who can attend?
The course will present new material to all FungiG members, ranging from master students to Ph.D. candidates, postdocs, and alumni professors working at Nanjing Agricultural University, Shanghai Jiao Tong University, Sun Yat-Sen University, Jiangsu Academy of Agricultural Sciences, and other universities. Students from the TU Wien master program “Biotechnology and Bioanalytics,” are welcome.
Students from the universities or academic institutions that are not listed above, please contact Irina Druzhinina.
Is the course free?
The WS2021 is free of charge but the number of places is limited.